Ovarian cancer is the sixth most common malignancy and the fifth most common cause of cancer-related death for women in the United States with an estimated 23,400 new cases and 13,900 deaths occurring in 2001. At Stony Brook University Medical Center, it accounted for 24 percent of all newly diagnosed gynecologic cancer cases in 2000, and ranked seventh in most frequent new cancers (Figure 2). The ovary can develop a variety of malignancies: 1) germ cell tumors from the ova; 2) sex cord stromal tumors from the follicles or stroma; 3) epithelial from the surface capsule; and 4) metastatic from numerous primary sites. Overall, 90 percent of ovarian malignancies are epithelial, the majority of which are serous histology. The other epithelial histological subtypes are mucinous, endometrioid, clear cell and Brenner tumors. Each of the histological subtypes can present as benign, borderline and malignant variants.
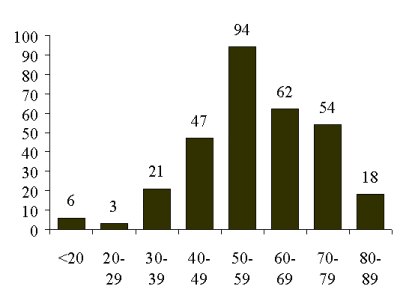
The majority of patients with epithelial ovarian cancer are postmenopausal, although it can occur at any age. The incidence increases with age with the peak incidence at 56 years and a decline after age eighty (Figure 3). The lifetime risk of developing epithelial ovarian cancer for a woman in the United States is approximately 1.4 percent. The majority of cases are sporadic with only 5-10% associated with a hereditary pattern. A genetic predisposition to epithelial ovarian cancer is associated with several mutations. The best characterized of these are BRCA-1 (chromosome 17, lifetime risk 28 to 44 percent), BRCA-2 (chromosome 13, lifetime risk 20 to 27 percent) and hMLHa and hMSH2 (mismatch repair genes associated with Hereditary Nonpolyposis Colorectal Cancer Syndrome, lifetime risk 10 percent).
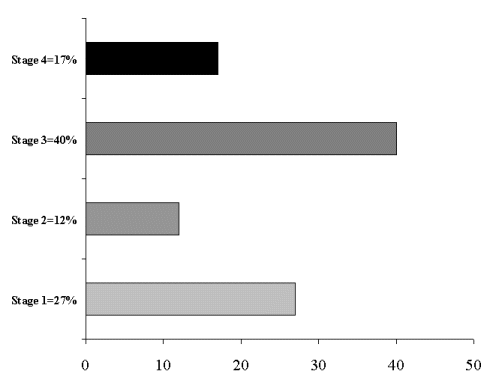
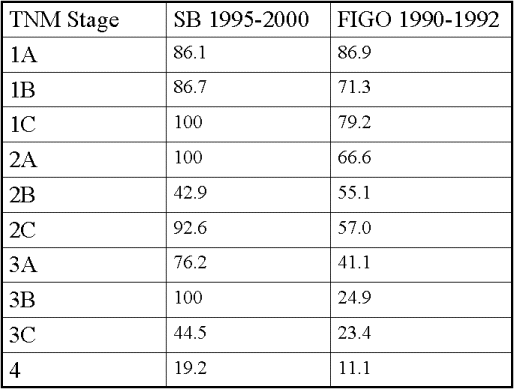
Epithelial ovarian cancer has the highest death to case ratio of the gynecologic malignancies. As women with early-stage disease do not have any distinct symptoms, the majority of women have advanced disease by the time they are diagnosed (Figure 4). This is unfortunate, as survival is inversely correlated with stage (Figures 5 and 6). In addition, population-based screening programs to detect early-stage disease with CA-125 blood testing and transvaginal ultrasound have not been effective and are not recommended. There are currently two large prospective screening studies underway: the National Institutes of Health's Prostatic, Lung, Colorectal and Ovarian Cancer study in the United States, and the Collaborative Trial of Ovarian Cancer Screening in the United Kingdom. The use of oral contraceptives for more than five years or undergoing bilateral tubal ligation is associated with a 50 percent reduction in the risk of developing epithelial ovarian cancer. Prophylactic bilateral oophorectomy also decreases the risk of developing epithelial ovarian cancer, although it may increase the risk of osteoporosis and heart disease. Furthermore, oophorectomy does not decrease the risk of developing primary peritoneal cancer.
The initial management of epithelial ovarian cancer is surgery with dual essential goals. The first goal is to provide accurate staging information for patients with apparently early-stage disease to assess the need for adjuvant therapy. The staging laparotomy should be performed through a vertical midline incision to provide access to the upper abdomen. Any free fluid should be aspirated, or peritoneal washings should be performed, and a sample sent for cytologic evaluation. A thorough visual inspection and palpation of all intraperitoneal surfaces should be performed. Any suspicious areas or adhesions should be biopsied. In the absence of any suspicious areas, multiple random biopsies should be performed, including the undersurface of the diaphragm. An omentectomy and pelvic and para-aortic lymph node sampling should be performed. In general, a total hysterectomy and bilateral salpingo-oophorectomy is performed, taking care to remove the adnexa intact. In young women who wish to preserve fertility, a unilateral adnexectomy and complete staging may be sufficient for low-risk disease. Thorough surgical staging is crucial as 10 to 30 percent of patients with apparent early-stage disease have metastatic disease on pathologic evaluation.
The second goal is to perform cytoreductive surgery for patients with advanced-stage disease. There are several benefits to cytoreductive surgery: 1) improving the patient's functional and nutritional status; 2) improving blood flow to smaller residual tumors which improves oxygenation and delivery of chemotherapeutic agents; 3) decreasing the likelihood of development of a chemoresistant clone; and 4) improving local immune function. Optimal cytoreduction is generally defined as residual disease less than 1 centimeter in maximum diameter. In order to achieve optimal cytoreduction, the surgeon must be intimately familiar with the pattern of spread of epithelial ovarian cancer as well as possess superb surgical skills. Frequently, optimal cytoreduction requires dissecting the retroperitoneal spaces, mobilizing the ureters, and bowel resection in the setting of extensive distortion by tumor. Survival is directly correlated with residual tumor volume with the longest survival in patients without macroscopic residual disease.
The need for adjuvant therapy is based upon the surgical findings. Approximately 25 to 30 percent of patients with epithelial ovarian cancer present with early-stage disease and are divided into low-risk and high-risk groups. Patients with low-risk disease are those with completely staged IA or IB (Figure 6) and grade 1 or 2 tumors (Figure 7). These patients have up to 95 percent disease-free survival and adjuvant therapy has not been shown to improve outcome. Patients with high-risk disease are those with completely-staged stage IC or IIA-C, grade 3, or clear cell histology tumors. These patients have up to 40 percent recurrence rates and adjuvant therapy has been shown to improve survival. Patients with advanced-stage disease have a risk of recurrence of 70 to 90 percent and adjuvant combination chemotherapy is the treatment of choice. In the United States, the standard regimen is six cycles of paclitaxel and platinum-based combination chemotherapy. The Gynecologic Oncology Group is attempting to determine the optimal number of cycles to administer in patients with early-stage disease.
The role of neoadjuvant chemotherapy with interval cytoreductive surgery is under investigation. Appropriate candidates are those with excessive surgical risks from concomitant medical disorders or poor nutritional status or those with clearly unresectable disease (e.g., porta hepatis, parenchymal liver or lung tumors). In these settings, the available data suggest that the outcome is at least comparable to initial surgical cytoreduction followed by adjuvant chemotherapy.
The options for patients who have achieved a complete clinical response include close surveillance, consolidative therapy, and second-look laparotomy or laparoscopy. To date, there are no definitive data suggesting that there is a survival benefit to any of these options. Typically, those patients who choose to undergo close surveillance are evaluated every three to six months with physical and pelvic examinations and CA-125 measurements (if their initial CA-125 level was elevated). A variety of consolidative therapeutic agents are available, including chemotherapy, hormonal therapy and radiotherapy. The Gynecologic Oncology Group and several pharmaceutical companies are sponsoring research trials of consolidative therapy. Second-look surgery is limited to those patients who have achieved a complete clinical response following initial therapy. The surgery may be performed via laparotomy or laparoscopy and includes careful exploration of the entire peritoneal cavity along with numerous biopsies. A second-look surgery provides prognostic information but does not improve survival. Thus, it should be performed selectively.
Unfortunately, the majority of patients with advanced-stage epithelial ovarian cancer will relapse. The choice of second-line chemotherapy is based upon the patient's initial responsiveness to platinum-based chemotherapy. Those patients who initially fail to respond are classified as having primary progressive disease and are best treated on a research protocol. Those patients who relapse within six months of treatment are classified as having platinum-resistant disease. This group of patients should be treated with non-cross-resistant agents with different mechanisms of action than the platinum-compounds. These agents include the topoisomerase-inhibitors (etoposide, topotecan), the antracyclines (doxorubicin, pegylated liposomal doxorubicin), the alkylators (cyclophosphamide, ifosphamide), and a variety of other agents (navelbine, gemcytabine, hexamethamelamine). Those patients who relapse greater than six months after treatment are classified as having platinum-sensitive disease and may be treated with further platinum-based chemotherapy. There is no evidence to suggest that combination chemotherapy offers any improvement over single-agent therapy in the relapsed setting. Overall, the response rates to chemotherapy in the second-line setting are 15 to 30 percent with response durations of 6 to 9 months. Ongoing studies are investigating the role of hormonal, biological and immunologic therapy.
Secondary cytoreductive surgery is limited to patients with platinum-sensitive recurrent disease with a disease-free interval of at least 12 months. Patients should have disease that appears to be confined to one or two locations and is potentially resectable. Survival is improved in this setting only if all macroscopic disease can be resected.
With aggressive surgical cytoreduction and modern combination chemotherapy, the prognosis for patients with epithelial ovarian cancer ranges from 20 to 100 percent at Stony Brook University Medical Center. These favorable results have been achieved through the efforts of a dedicated team of gynecologic oncologists, pathologists, radiologists and support staff which includes nurses, physical therapists, nutritionists and social workers.
Ovarian Cancer Summary by Michael L. Pearl, MD, FACOG, FACS, Assistant Professor of Obstetrics, Gynecology and Reproductive Medicine and Surgery.
Age at Diagnosis in 305 Patients With Ovarian Cancer at Stony Brook University Medical Center 1995 - 2000.
Stage at Diagnosis in Patients With Ovarian Cancer at Stony Brook University Medical Center 1995 - 2000, Classified Using the American Joint Committee (AJCC) TNM System.
Relative Survival Rates in Patients With Ovarian Cancer at Stony Brook University Medical Center 1995-2000 by Stage at Diagnosis Compared With American College of Surgeons Commission on Cancer National Cancer Data Base (NCDB) Data.
| TNM Stage | Stony Brook | NCDB |
| Stage 1 | 87% | 84% |
| Stage 2 | 84% | 63% |
| Stage 3 | 33% | 29% |
| Stage 4 | 13% | 17% |
Overall Adjusted Survival by Stage at Diagnosis in 298 Cases of Ovarian Cancer at Stony Brook University Medical Center Compared With Results Published by International Federation of Obstetricians and Gynecologists (FIGO)
Histologic Grade Differentiation in Ovarian Cancers 1995 - 2000 at
Stony Brook University Medical Center

- Cancer Facts and Figures, 2001. American Cancer Society, Atlanta, GA.
- Cancer Incidence and Mortality in New York State, Volume 1. Cancer Incidence and Mortality by County, 1994-1998, New York State Department of Health, New York State Cancer Registry, December 2000, Albany, NY.
- SEER Surveillance, Epidemiology and End Results SEER Cancer Incidence Public-Use Database 1973-1997 NCI CD-ROM, National Cancer Institute, US. Department of Health and Human Services, Cancer Statistics Branch, Bethesda, MD.
- Manual For Staging of Cancer, 5th Edition, American Joint Committee on Cancer, 1997, J.B. Lippincott Company, Philadelphia, PA.
- National Cancer Data Base Annual Review of Patient Care 1998, CANCER Journal of the American Cancer Society, June 2000, Atlanta, GA.
- Standards of the Commission on Cancer, Volume I: Cancer Program Standards, 1995, Chicago, IL.
- Journal of Epidemiology and Biostatistics: FIGO Annual Report on the Results of Treatment in Gynaecological Cancer 23rd volume, Milan, Italy.


