Example #1
SBCC Investigator and Program:
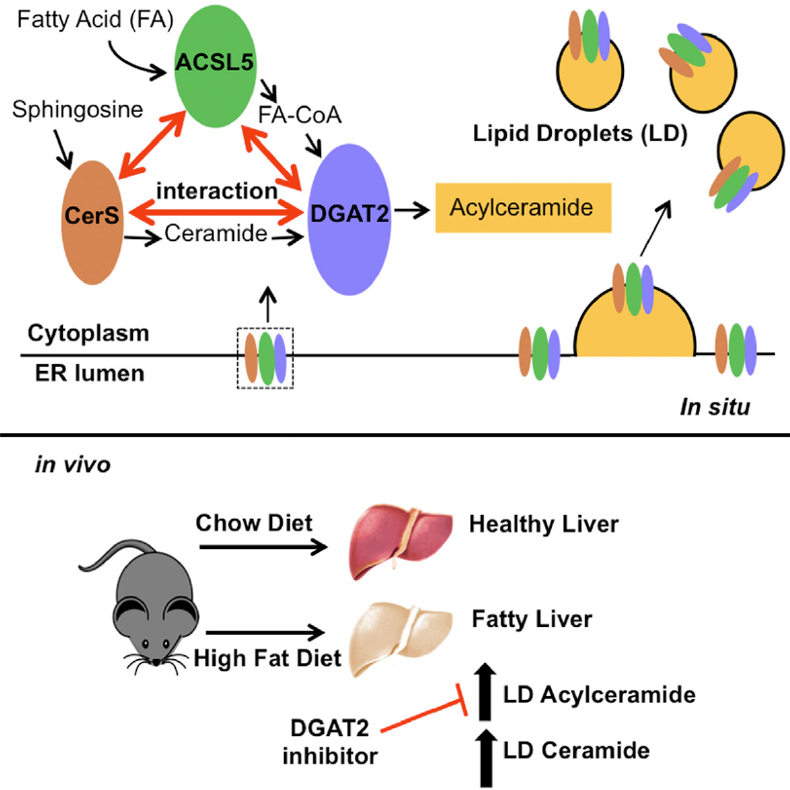
L. Obeid (LSMC), Y. Hannun (LSMC) Publication: Ceramide is Metabolized to Acylceramide and Stored in Lipid Droplets. Cell Metabolism, 2017, PMCID PMC5472424; R01 GM097741-22.
Significance of Findings: Pioneering work by Obeid and Hannun led to the discovery of ceramide as a bioactive lipid and its roles in stress responses and apoptosis. In this study, their groups defined a novel pathway for ceramide metabolism by which ceramide is acylated by the action of DGAT (diacylglycerol acyl transferase, which also synthesized triacylglycerol). This results in ‘storage’ of ceramide as acyl ceramide in lipid droplets. They went on to show that the pathway attenuates the growth suppressing functions of ceramide. Reciprocally, inhibition of DGATs augments the actions of ceramide and chemotherapeutic agents.
MS-SR Utilization: Studies in the MS-SR were critical in dissecting the ceramide metabolism and identifying acylceramide and in measuring components of this novel pathway. Identification of acylceramide led to the establishment of this lipid and its biologic significance.
Example #2
SBCC Investigators and Programs: H. Kim (ODMC), M. Seeliger (ODMC), J. Haley (LSMC)
Publication: Prolyl isomerization of FAAP20 catalyzed by PIN1 regulates the Fanconi anemia pathway, PLOS Genetics 2019. PMCID: PMC6400411; R01CA218132
Significance of Findings: The Fanconi Anemia (FA) pathway is a multi-step DNA repair process at stalled replication forks in response to DNA interstrand cross-links (ICLs). Pathological mutation of key FA genes leads to the inherited disorder FA, characterized by progressive bone marrow failure and cancer predisposition. Mono-ubiquitination FANCD2, a key component of the FA pathway, by the FA core complex is an essential gateway that connects upstream DNA damage signaling to enzymatic steps of repair. FAAP20 is a key component of the FA core complex, and regulated proteolysis of FAAP20 mediated by the ubiquitin E3 ligase SCFFBW7 is critical for maintaining the integrity of the FA complex and FA pathway signaling. However, upstream regulatory mechanisms that govern this signaling remain unclear. SBCC investigators showed that PIN1, a phosphorylation-specific prolyl isomerase, regulates the integrity of the FA core complex, thus FA pathway activation. They demonstrated that PIN1 catalyzes cis-trans isomerization of the FAAP20 pSer48-Pro49 motif and promotes FAAP20 stability. Mechanistically, PIN1-induced conformational change of FAAP20 enhances its interaction with the PP2A phosphatase to counteract SCFFBW7-dependent proteolytic signaling at the phosphorylated degron motif. Accordingly, PIN1 deficiency impairs FANCD2 activation and the DNA ICL repair process. Together, this study establishes PIN1-dependent prolyl isomerization as a new regulator of the FA pathway and genomic integrity.
MS-SR Utilization: Measurement of FAAP20 phosphorylation sites using mass spectrometry were central parts of the project and critical to this publication.
Example #3
SBCC Investigators and Programs: J. Haley (MS-SR and LSMC), M. Garcia-Diaz (ODMC)
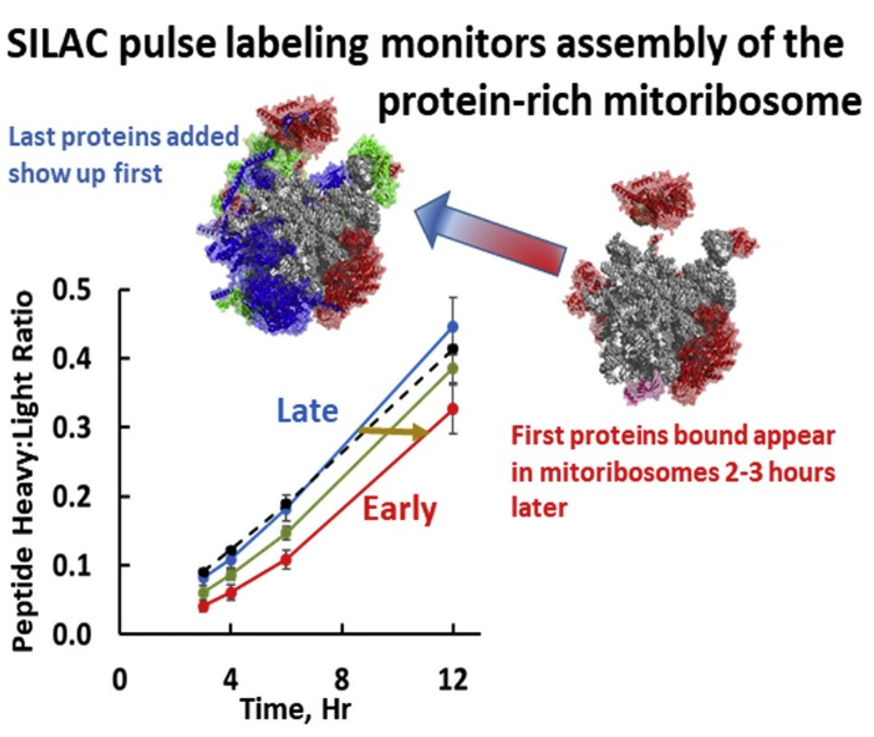
Publication: Kinetics and Mechanism of Mammalian Mitochondrial Ribosome Assembly. Cell Reports, 2018, PMCID: PMC5855118; 1R01GM112790.
Significance of Findings: Mutation and dysfunction of mitochondrial proteins can promote oncogenesis and tumor progression. Mammalian mtDNA encodes only 13 proteins, all essential components of respiratory complexes, synthesized by dedicated mitochondrial ribosomes. Mitoribosomes contain greatly truncated RNAs transcribed from the mtDNA and include a structural tRNA in place of 5S RNA as a scaffold for binding 82 nucleus-encoded proteins, MRPs. Recent cryoEM studies have determined the structure of the mitoribosome, but its mechanism of assembly is unknown but it is appreciated that assembly is a slow process initiated at the mtDNA nucleoid. The MS-SR conducted SILAC pulse-labeling experiments providing a basis for distinguishing MRPs that bind at early and late stages in mitoribosome assembly, forming the basis of a working model for mitoribosome assembly. MRPs that are tightly associated in the structure frequently join the complex in a coordinated manner, possibly as pre-formed modular subassemblies. Clinically significant MRP mutations reported to date affect proteins that bind early on during assembly. A follow-up study of mitochondrial changes associated with cancer epithelial and mesenchymal (EMT) phenotypes is in progress, where SILAC quantitation of proteomic datasets are essential.
MS-SR Utilization: The time-dependent incorporation of labeled amino acids (SILAC) and mass spectrometry was a central part of the project and critical to this publication.
Example #4
SBCC Investigator and Program: D. Canals (LSMC), J. Haley (MS-SR and LSMC) and Y. Hannun (LSMC).
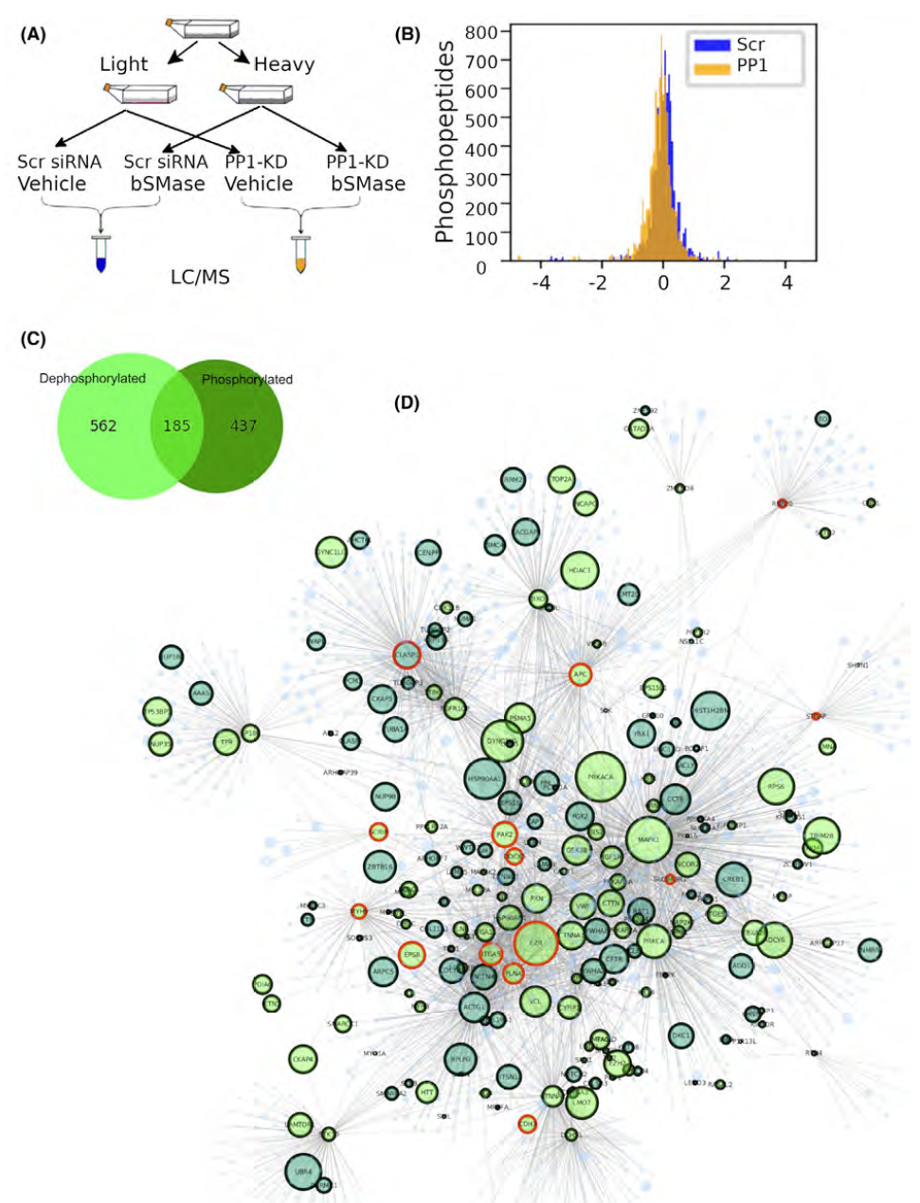
Publication: The doxorubicin-induced cell motility network is under the control of the ceramide-activated protein phosphatase 1 alpha. FASEB J, 2021 (in press).
Significance of Findings: Work from collaborations among several members of the LSMC had resulted in identifying a rich complexity in ceramide function and metabolism that were enabled by the utilization of advanced LC/MS-MS approaches in the MS-SR. Biologic investigation suggested that different molecular species of ceramide result in distinct functions, probably defined by the specific pathways in generating ceramide and their subcellular localization. This team had also recently reported that a specific pool of ceramide, located in the plasma membrane, mediated the effects of sublethal doses of the chemotherapeutic compound doxorubicin on enhancing cancer cell migration. They also identified neutral sphingomyelinase2 (nSMase2) as the enzyme responsible to generate this bioactive pool of ceramide. In this work, they defined the mechanisms of action of ceramide. Using an elaborate phosphoproteomic approach, they identified protein phosphatase 1 alpha isoform (PP1 alpha) as the specific PP1 isoform to mediate this phenotype in response to ceramide generated at the plasma membrane. Coupled with bioinformatic approaches, this led to the identification of several ceramide-PP1 alpha downstream substrates. Studies on phospho mutants of ezrin (T567) and Scrib (S1378/S1508) demonstrated that their dephosphorylation is sufficient to enhance cell migration. In summary, they identified a mechanism whereby reduced doses of doxorubicin result in the dysregulation of cytoskeletal proteins and enhanced cell migration, which could explain the reported effects of doxorubicin worsening cancer metastasis in animal models.
MS-SR Utilization: Initial studies in the MS-SR were critical in dissecting the complexity of the ceramide species. This work was specifically dependent on the advanced proteomic and phosphoproteomic capabilities of the MS-SR.