Stereotactic Body Radiation Therapy (SBRT) is a technique that delivers high radiation doses to a tumor target of ant body sires, just like radiosurgery of the brain. It is delivered in a few treatments but using a high dose per treatment. The schedule is usually two to five sessions over a one- to two-week period.
Stony Brook University Hospital has world-renowned leading experts in SBRT. There is a large volume of published data demonstrating dramatically higher rates of local control with SBRT when compared to conventional radiotherapy. SBRT can deliver an optimal radiation dose to both new primary and metastatic cancer sites. It can also be used on previously irradiated sites safely to avoid damage to the critical structure, such as the spinal cord. SBRT is used in the treatment of spinal tumors, liver tumors, and other sites as well. Treating lung cancer with SBRT is an excellent alternative choice for patients with medically inoperable tumors, elderly patients who are considered high risk for surgery, or patients who refuse surgical treatment.
The Varian® On-Board Imager® (OBI) is an automated patient positioning system for image-guided radiation therapy (IGRT). The OBI kV imaging system delivers improved tumor targeting using high resolution, low-dose digital imaging in the treatment room. It provides x-ray position verification in seconds, enabling the precise patient setup. With this system, Stony Brook's Department of Radiation Oncology is equipped to offer the specialized Image-Guided Radiation Therapy (IGRT) treatment called Stereotactic Body Radiation Therapy (SBRT).
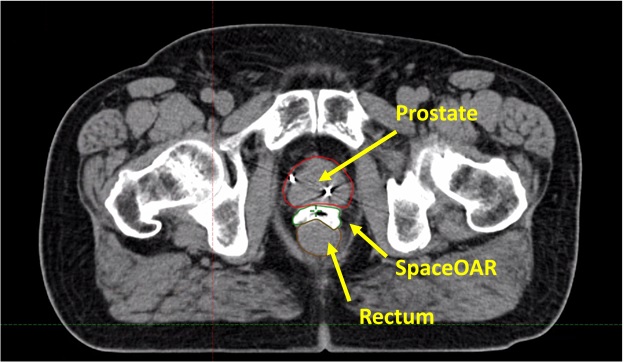
SpaceOAR for SBRT Prostate Treatment
SpaceOAR hydrogel is an option for men who undergo radiation treatment for prostate cancer. It acts as a spacer providing space between the rectum and the prostate, making it much less likely that the rectum is exposed to radiation. It is injected into place prior to the start of radiation treatment. Patients may be awake or asleep under general anesthesia for the procedure. SpaceOAR hydrogel is minimally invasive, remains stable during radiation therapy and then is gradually absorbed by the body after radiation therapy has been completed.
Clinical trials in Europe and the U.S. have demonstrated that the hydrogel is safe and that the space created significantly reduces the radiation delivered to the rectum. The randomized SpaceOAR hydrogel U.S. Clinical Trial found that patients who received the hydrogel spacer reported significantly less rectal pain during radiotherapy and had significantly less severe long-term rectal complications.